NEWS FROM THE YEAR
-
NLM will update AccessGUDID to include more Global Medical Device Nomenclature (GMDN) information
Posted: August 21, 2023
Updated: On August 14, 2023, AccessGUDID data updated with more Global Medical Device Nomenclature (GMDN) information.
Posted: July 19, 2023
NLM will update AccessGUDID to include more Global Medical Device Nomenclature (GMDN) information - Term Codes, Code Status (Active or Obsolete), and a GMDN Implantable flag (True or False). Device records containing a GMDN Preferred Term (gmdnPTName) will include the equivalent GMDN Term Code on AccessGUDID.<gmdnTerms> <gmdn> <gmdnCode>43691</gmdnCode> <gmdnPTName>Bare-metal biliary stent</gmdnPTName> <gmdnPTDefinition>A non-bioabsorbable tubular device intended ... </gmdnPTDefinition> <implantable>True</implantable> <gmdnCodeStatus>Active</gmdnCodeStatus> </gmdn> </gmdnTerms>
This update is intended to provide the end user with enhanced search and retrieval capabilities for AccessGUDID data. In addition to the GMDN Preferred Term and Definition searching that is already available, users will be able to use the AccessGUDID Advanced Search function to retrieve device records by searching with GMDN Term Code and GMDN Term Status.
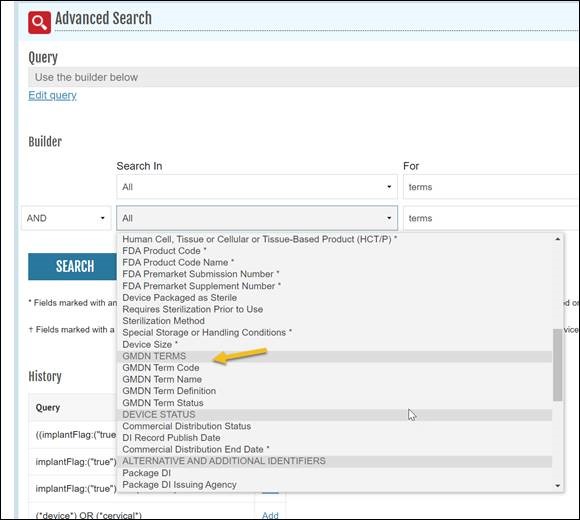
The AccessGUDID API will also be updated to version 3 (v3) to incorporate this new information. The Device Lookup and Device History functions will include the three new GMDN fields in their output. Previous versions (v1 and v2) of the AccessGUDID API remain unchanged and will be available as noted in the API documentation.
Device labelers can contact the FDA UDI Help Desk for more information and find resources on the GMDN website to identify alternatives to obsolete term codes.
Questions about the new GDMN data fields and their implementation on the AccessGUDID API and searching can be directed to the NLM Help Desk .
-
AccessGUDID API changes to include UMLS API authentication using API Key
Posted: March 1, 2023
AccessGUDID API changes to include UMLS API authentication using API Key
AccessGUDID API calls for SNOMED information have been updated to accept a UMLS API Key for authentication.
This change reduces the burden on users, with the new option requiring a single API call to retrieve data instead of multiple API calls.
The two AccessGUDID APIs affected by this are the Device SNOMED API and the Implantable List API .
The change for AccessGUDID would be switching the ST parameter to a new apiKey parameter.
Here are a few examples of these APIs with either single-use ticket or API Key :
Device SNOMED:
-
Single-use ticket:
https://accessgudid.nlm.nih.gov/api/v2/devices/snomed?ticket={ST}&di=08717648200274
-
API Key:
https://accessgudid.nlm.nih.gov/api/v2/devices/snomed?apiKey={API_KEY}&di=08717648200274
Implantable List:
-
Single-use ticket:
https://accessgudid.nlm.nih.gov/api/v2/devices/implantable/list.json?ticket={ST}&page=30&per_page=2
-
API Key:
https://accessgudid.nlm.nih.gov/api/v2/devices/implantable/list.json?apiKey={API_KEY}&page=30&per_page=2
Implantable List Download:
-
Single-use ticket:
https://accessgudid.nlm.nih.gov/api/v2/devices/implantable/download?ticket={ST}
-
API Key:
https://accessgudid.nlm.nih.gov/api/v2/devices/implantable/download?apiKey={API_KEY}
-
Single-use ticket:
-
Upcoming Changes to Public IP Addresses for AccessGUDID
Posted: June 28, 2019
Update 8/2/2019 - AccessGUDID beta is live, please visit https://accessgudid-beta.nlm.nih.gov/
Update 8/29/2019 - AccessGUDID beta will be promoted to production on September 4, 2019.
Update 9/4/2019 - AccessGUDID beta now in production, please visit https://accessgudid.nlm.nih.gov/
We will be adding two new data centers with new IP addresses for AccessGUDID. We expect these data centers to be operational in beta mode around the first week of August 2019 and in production mode around the first week of September 2019.
If your organization uses firewall rules for accessing AccessGUDID, you need to update your firewall rules to allow for these additional IP addresses.
If you have any concerns or issues, please contact NLM Support Center.
Datacenter-1 (Current - since 2015)
IPV4: 130.14.16.152
IPV6: 2607:f220:41e:1016::152
Datacenter-2 (Current - since 2015)
IPV4: 165.112.140.152
IPV6: 2607:f220:41f:1140::152
Datacenter-3 (New - around first week of August 2019, Now Live - September 4, 2019)
IPV4: 3.94.60.161
IPV4: 3.217.176.226
Datacenter-4 (New - around first week of August 2019, Now Live - September 4, 2019)
IPV4: 52.36.232.238
IPV4: 54.203.164.238 -
GUDID "Grace Period" Reduced from 30 Days to 7 Days
Posted: March 11, 2019
Today, the "grace period" for all GUDID records was changed from 30 days to 7 days. After a GUDID record’s Publish Date passes, the record enters its grace period. During the grace period, the record remains nonpublic and editable by the author. After the grace period ends, the record appears publicly on AccessGUDID.
Starting March 11, 2019 all records 7 days past their Publish Date will appear on AccessGUDID.
For more information, please visit FDA's GUDID Enhancements and Fixes page. -
AccessGUDID V2 APIs Promoted From Beta to Production
Posted: December 28, 2018
Version 2 (V2) APIs will be promoted from beta to production status on January 1, 2019. If you are using our V1 APIs, we encourage you to update your code to use the V2 APIs. After December 31, 2019, the V1 APIs will be deprecated and no longer available for use. If you have questions about the migration to V2, please contact us via the NLM Support Center.
For more information about the V2 APIs, please visit the API Documentation. To learn more about newly added data elements to the V2 APIs, please refer to the new schema published on April’2018. -
TLS1.0 Protocol Disabled as of September 15, 2018
Posted: September 17, 2018
TLS1.0 protocol reached its end of life on June 30th 2018. NLM across all its public websites is disabling TLS1.0 protocol by September 15, 2018. If you have any questions please let us know by contacting the NLM Support Center. -
FDA Premarket Submission/Supplement Numbers Available for Some Devices
Posted: July 9, 2018
As of July 9, FDA Premarket Submission Numbers and Supplement Numbers are part of the device record. They can be found in web search and download files. Not every device record contains premarket numbers. If premarket numbers are available and publicly releasable, they appear in the device record. -
New: Download Large Sets of 10,000+ Records. Coming Soon: Record Versions
Posted: February 8, 2018
Coming Soon: More easily track data changes and sync your database to the latest data available on AccessGUDID. And more easily link GUDID data to other data sets! Starting April 2, 2018, we plan to add new GUDID data elements to the download files and APIs. Review the new schema and get prepared!
Have you ever tried to export search results and hit the 10,000 record limit? A new option is to download a pre-built (canned) query—see what’s available here. And remember that you can always download the full data set and import it into your own database to filter and sort according to your needs.- Public Version Number
- Public Version Date
- Public Version Status (new/update/delete)
- Public Device Record Key
- Labeler D-U-N-S® Number
- FDA Premarket Submission Number (to be added April 2, 2018 but will not contain any values until Summer 2018)
- FDA Premarket Supplement Number (to be added April 2, 2018 but will not contain any values until Summer 2018)
-
New: Output Full Records in Linkable Tables
Posted: June 1, 2017
Do you want AccessGUDID data in tables that can be easily linked? Or do you want to export your search results as full records that include all fields?When downloading the full data set or exporting your search results, choose the delimited text file option. These text files can be imported into Excel for easy viewing. They can also be imported into Access, Oracle, MySQL, or another relational database application, where you can link these tables to easily query and manipulate the data. Detailed instructions are available here. Bulk import scripts for Oracle and MySQL are also available.
-
Update to GUDID Download File
Posted: March 17, 2017
The GUDID data element “Package Type” has now been added to the download file. Package Type provides the text to describe the outer packaging of the product and enables users to understand higher level packaging configurations. For more information on GUDID Data Elements, please see the GUDID Data Elements Reference Table. Please download the updated schema. This data element will soon be available under AccessGUDID search as well. -
New SNOMED CT API in Beta
Posted: July 7, 2016
The Device SNOMED API accepts a DI or UDI and returns the SNOMED CT name and identifier associated with the device. A UMLS single-use ticket is required. User testing and feedback are welcome! Please contact customer support with your comments. -
AccessGUDID in Production!
Posted: March 22, 2016
AccessGUDID is officially production ready. The site has expanded from basic search and download services to offer advanced search, RSS feeds, and APIs. Thank you for your feedback while the site was in beta! -
New UDI Parser
Posted: February 16, 2016
The new Parse UDI API separates a UDI into its component parts, which can include:- DI
- Manufacturing date
- Expiration date
- Lot/batch number
- Serial number
- Donation identification number (if applicable)
-
AccessGUDID changing to HTTPS
Posted: December 1, 2015
AccessGUDID is changing its base URLs from HTTP to HTTPS.
The new base URL for the web application is: https://accessgudid.nlm.nih.gov/
The new base URL for the web service is: https://accessgudid.nlm.nih.gov/api/v1/devices/
If you are using our web services with HTTP, please update your code to use HTTPS by January 31, 2016. After that date, HTTP access will be disabled. -
New APIs and Download Exit Beta
Posted: November 16, 2015
The Lookup API, Implantable API and Implantable Download have completed their beta testing period! Visit the API Documentation page to learn more about these features. -
AccessGUDID Full Release File Notification
Posted: October 26, 2015
Due to data errors, the Full Release file for the month of October has been removed. An updated file will be available on Monday, November 2, 2015. We apologize for the inconvenience. -
New APIs and Download
October 19, 2015
Two new APIs and a new download are now available:
- The Lookup API returns all AccessGUDID fields for a single device record.
- The Implantable API and Implantable Download return a list of implantable devices that have been submitted to AccessGUDID. The API was developed specifically to meet the Implantable Device List specifications in the ONC EHR Certification and CMS MU3 Final Rules.
These new features are available in beta release for a brief user testing period before their full release. We encourage you to test them out and share your feedback. Visit the API Documentation page to find out more!
-
AccessGUDID Release Files Notification
Posted: August 13, 2015
Update 8/19/2015 - GUDID functionality has been restored and a new full release is available
The FDA identified a security vulnerability in GUDID, and decided to take that system offline until a patch is implemented. We do not expect full GUDID functionality to be available until at least August 20, 2015. However, the Pre-production & Production system HL7 SPL submission option has been restored. We will provide updates via the FDA UDI website and through GovDelivery notifications.
No database release files will be posted to AccessGUDID while GUDID is offline. The next downloadable file will be available after GUDID’s full functionality has been restored and will be a full release. -
New Video: Search and Download
Posted: July 2, 2015
Check out the Help page to watch a short demo of how to use Search and Download on AccessGUDID. This video gives a quick overview of some of the main features of AccessGUDID and some helpful tips on how to search. -
Daily Release files notification
Posted: June 2, 2015
Some of the Daily Release update files during the month of May are missing a few device record updates. If you used the Daily Release update files during the month of May, we recommend you download the current Full Release file to ensure you have the most updated information. -
Launch of AccessGUDID
Posted: May 4, 2015
Launch of the new AccessGUDID Website!

